Abstract
Introduction: Generation of BCR-ABL fusion gene by reciprocal translocation of chromosomes 9 and 22 immortalizes hematopoietic stem cells by mechanisms such as activation of the JAK-STAT pathway, translational activation of BCL-XL, and inhibition of DNA repair, thereby leading to chronic myeloid leukemia (CML). Amazing improvement in the prognosis of CML has been achieved since the introduction of tyrosine kinase inhibitors (TKIs). Imatinib, a 1st-generation TKI, and dasatinib and nilotinib, 2nd-generation TKIs, are generally used for chronic phase (CP) CML as induction therapy. However, to date, no consensus about the cessation of TKIs in CP-CML patients has been obtained. We recently reported the case of a CP-CML patient with long-term complete molecular response (MR) after cessation of dasatinib, who has been maintaining memory CTLs with T cell receptor (TCR) clonality (Jo et al. Oncology Letters 15: 2935-2938, 2018). Here, we show that up-regulation of memory CTLs occurs early in dasatinib-treated patients compared with those treated with other TKIs.
Methods: We examined the TCR V beta gene repertoire to analyze TCR clonality of CD8-positive T cells in TKI-treated CP-CML patients using flow cytometry.
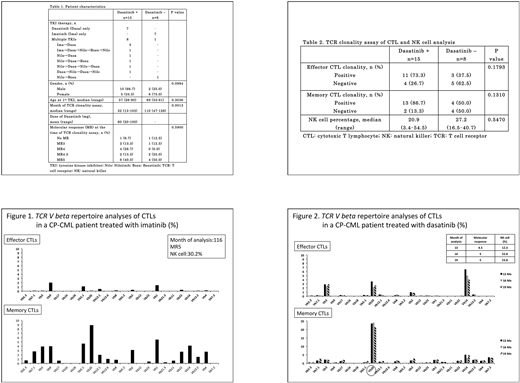
Results: Table 1 summarizes the data comparing patients treated with TKIs including dasatinib (Dasa group) and those treated with TKIs without dasatinib (non-Dasa group). Seven patients were treated with dasatinib only; 7, with imatinib only; 8, with multiple TKIs, including dasatinib; and 1, with multiple TKIs without dasatinib. The median age at first TKI administration was 57 years in the Dasa group and 69 years in the non-Dasa group. No significant statistical difference was observed in age at first TKI administration. The time of TCR clonality assay was significantly earlier in the Dasa group than in the non-Dasa group (P = 0.0013). There was no significant difference in the MR at the time of TCR clonality assay between the 2 groups. Figure 1 shows representative data of the TCR clonality assay of the patients in the non-Dasa group. We defined a TCR V beta gene percentage of 10% and above as being positive for monoclonality in this study. The time of analysis was at 116th month (Mo) after the 1st imatinib administration, and NK cell percentage was 30.2% at that time. TCR monoclonality was observed in neither effector CTLs (upper panel) nor memory CTLs (lower panel), although the patient had gained MR5. Figure 2 shows representative time-course data of the patients in the Dasa group. MR levels were MR4.5 (13th Mo), MR5 (16th Mo), and MR5 (19th Mo). Interestingly, memory CTL clonality with the TCR V beta 20 gene had already been observed in the 13th Mo, and it had been continuously observed in the 16th and 19th Mo. NK cell percentages were less than 24% throughout the observation period. Table 2 summarizes the CTL clonality assay results and NK cell percentages. There was no significant change in the NK cell percentages between the 2 groups. Although no statistical significance was observed in both effector and memory CTL clonality between the 2 groups, it is notable that approximately 73% and 87% positivity of effector and memory CTL clonality was observed in the Dasa group. Approximately 38% and 50% positivity of effector and memory CTL clonality was observed in the non-Dasa group, although the TKI exposure time for this group was significantly longer. Notably, the positive percentages of effector and memory CTL clonality in the non-Dasa group are quite similar to the overall probabilities of maintenance of deep MR reported in various imatinib-stop studies such as the STIM study (Mahon et al. Lancet Oncol 11: 1029-1035, 2010) and the TWISTER study (Ross et al. Blood 122: 515-522, 2013). These results suggest that acquisition of CTL clonality may correlate with treatment-free remission (TFR) in CP-CML patients treated with TKIs.
Conclusions: Effector and memory CTL clonality was attained more rapidly and frequently in dasatinib-treated CP-CML patients than in patients treated with TKIs without dasatinib. There was no significant difference in the NK cell percentages. The positive percentages of CTL clonality resembled the percentages of TFR in various TKI-stop studies. These results suggest that the acquisition of CTL clonality may provide long-term remission and TFR to CP-CML patients and that cessation of TKIs should be considered in patients with clonal expansion of memory CTLs, irrespective of NK cells.
Jo:Bristol-Myers Squibb: Honoraria.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal